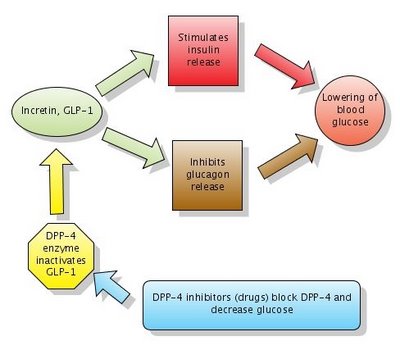
Action of DPP-4 inhibitors. Note that DPP-4 normally inactivates GLP-1. DPP-4 inhibitors block DPP-4 which in turn leaves GLP-1 active. Click to enlarge the figure. Created with Gliffy.
What is Glucagon-like peptide-1 (GLP-1)?
Glucagon-like peptide-1 (GLP-1) is a GI peptide that stimulates insulin secretion (similar to sulfonylureas). GLP-1 also inhibits glucagon release, gastric emptying and food absorption. GLP-1 and another similar peptide are called incretins. As noted above, incretins have a dual action which leads to lowering blood glucose:
1. Stimulate insulin release
2. Inhibit glucagon release
Exenatide (Byetta) is a GLP-1 receptor agonist approved for adjunctive therapy for patients with DM 2 who are not well controlled on oral agents. It is available only as injections and has to be administered twice daily.
DPP-4 inhibitors, or gliptins, increase GLP-1 levels by blocking the enzyme which inactivates GLP-1. The enzyme is called DPP-4 (dipeptidyl peptidase-4). They act similarly to Byetta (see figure above) but have the big advantage to be available in oral form (pills). Gliptins used for treatment of DM2 include sitagliptin (Januvia) and vildagliptin (Galvus).
What is Liraglutide?
Liraglutide (Victoza) is a long-acting glucagon-like peptide-1 (GLP-1) analog that was developed by Novo Nordisk for the treatment of type 2 diabetes. Liraglutide has a half-life after subcutaneous injection of 11–15 hours, making it suitable for once-daily dosing (in contrast to Byetta's twice daily).
Liraglutide. Image source: Wikipedia, public domain.
Liraglutide (Victoza) is a long-acting glucagon-like peptide-1 (GLP-1) analog that was developed by Novo Nordisk for the treatment of type 2 diabetes. Liraglutide has a half-life after subcutaneous injection of 11–15 hours, making it suitable for once-daily dosing (in contrast to Byetta's twice daily).

Liraglutide. Image source: Wikipedia, public domain.
Liraglutide (Victoza) superior to sitagliptin (Januvia) for reduction of HbA1c in diabetics
This Lancet study assessed the efficacy and safety of the human GLP-1 analogue liraglutide versus the DPP-4 inhibitor sitagliptin, as adjunct treatments to metformin, in individuals with type 2 diabetes who did not achieve adequate glycaemic control with metformin alone.
More than 600 participants (aged 18—80 years) with type 2 diabetes mellitus who had inadequate glycaemic control (glycosylated haemoglobin [HbA1c] 7·5—10·0%) on metformin (more than 1500 mg daily) were enrolled.
Participants were randomly allocated to receive 26 weeks' treatment with 1·2 mg or 1·8 mg subcutaneous liraglutide once daily, or 100 mg oral sitagliptin once daily.
Greater lowering of mean HbA1c (8·5% at baseline) was achieved with 1·8 mg liraglutide (−1·50%) and 1·2 mg liraglutide (−1·24%) than with sitagliptin (−0·90%).
Nausea was more common with liraglutide (27%) on 1·8 mg. Minor hypoglycaemia was recorded in about 5% of participants in each treatment group.
Liraglutide was superior to sitagliptin for reduction of HbA1c, and was well tolerated with minimum risk of hypoglycaemia. These findings support the use of liraglutide as an effective GLP-1 agent to add to metformin.
More than 600 participants (aged 18—80 years) with type 2 diabetes mellitus who had inadequate glycaemic control (glycosylated haemoglobin [HbA1c] 7·5—10·0%) on metformin (more than 1500 mg daily) were enrolled.
Participants were randomly allocated to receive 26 weeks' treatment with 1·2 mg or 1·8 mg subcutaneous liraglutide once daily, or 100 mg oral sitagliptin once daily.
Greater lowering of mean HbA1c (8·5% at baseline) was achieved with 1·8 mg liraglutide (−1·50%) and 1·2 mg liraglutide (−1·24%) than with sitagliptin (−0·90%).
Nausea was more common with liraglutide (27%) on 1·8 mg. Minor hypoglycaemia was recorded in about 5% of participants in each treatment group.
Liraglutide was superior to sitagliptin for reduction of HbA1c, and was well tolerated with minimum risk of hypoglycaemia. These findings support the use of liraglutide as an effective GLP-1 agent to add to metformin.
References:
Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. The Lancet, Volume 375, Issue 9724, Pages 1447 - 1456, 24 April 2010.
No comments:
Post a Comment